BACKGROUND
Early prognosis of dengue virus (DENV) an infection can enhance clinical outcomes by guaranteeing shut follow-up, initiating acceptable supportive therapies and elevating consciousness to the potential of hemorrhage or shock. Non-structural glycoprotein-1 (NS1) has confirmed to be a helpful biomarker for early prognosis of dengue.
A quantity of rapid diagnostic tests (RDTs) and enzyme-linked immunosorbent assays (ELISAs) focusing on NS1 antigen (Ag) are actually commercially out there.
Here we evaluated these tests using a well-characterized panel of clinical samples to find out their effectiveness for early prognosis.
RESULTS
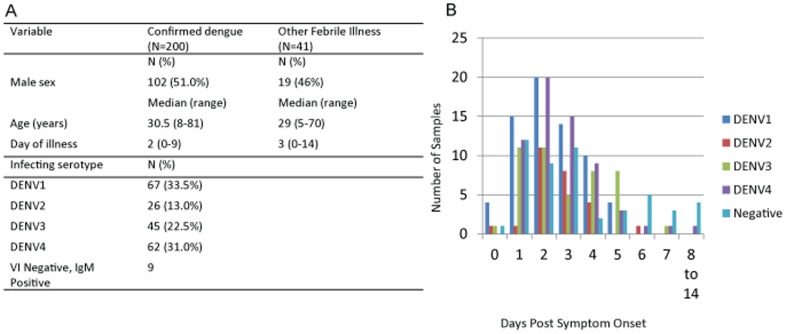
Retrospective samples from South America had been used to judge the next tests: (i) “Dengue NS1 Ag STRIP” and
(ii) “Platelia Dengue NS1 Ag ELISA” (Bio-Rad, France), (iii) “Dengue NS1 Detect Rapid Test (1st Generation)” and
(iv) “DENV Detect NS1 ELISA” (InBios International, United States), (v) “Panbio Dengue Early Rapid (1st era)”
(vi) “Panbio Dengue Early ELISA (2nd era)” and (vii) “SD Bioline Dengue NS1 Ag Rapid Test” (Alere, United States).
Overall, the sensitivity of the RDTs ranged from 71.9%-79.1% whereas the sensitivity of the ELISAs assorted between 85.6-95.9%, using virus isolation because the reference methodology.
Most tests had decrease sensitivity for DENV-Four relative to the opposite three serotypes, had been much less delicate in detecting secondary infections, and gave the impression to be most delicate on Day 3-Four submit symptom onset.
The specificity of all evaluated tests ranged from 95%-100%.
CONCLUSIONS
ELISAs had better general sensitivity than RDTs. In conjunction with different parameters, the efficiency information can assist decide which dengue diagnostics needs to be used through the first few days of sickness, when the sufferers are more than likely to current to a clinic looking for care.
Application of recombinant Echinococcus granulosus antigen B to ELISA kits for diagnosing hydatidosis.
Echinococcus granulosus causes human cystic echinococcosis as an essential public well being drawback in lots of areas of the world. There are some issues in major prognosis akin to cross-reaction with sera from sufferers with different parasitic illness in serological tests.
The use of an acceptable supply of antigenic materials is a vital and essential level within the enchancment of the serodiagnostic options akin to enzyme-linked immunosorbent assay (ELISA) methodology.
We expressed and purified recombinant AgB of Echinococcus granulosus and used as antigen in ELISA methodology.
Serum samples got from 36 cystic hydatid illness sufferers which have been confirmed by surgical treatment in addition to 36 wholesome people sera had been examined by ELISA methodology using recombinant AgB and in contrast with business package (Euroimmun) for specificity and sensitivities worth.
The sensitivity of 91.66% and specificity of 97.22% had been decided by do-it-yourself package.
