OBJECTIVETissue transglutaminase (tTG) is a serious autoantigen recognised by IgA anti-endomysial antibodies (IgA EMA).
Enzyme linked immunosorbent assays (ELISA) for IgA anti-tissue transglutaminase antibodies (IgA tTG) have due to this fact been developed in its place serological screening take a look at to IgA EMA for coeliac illness (CD).
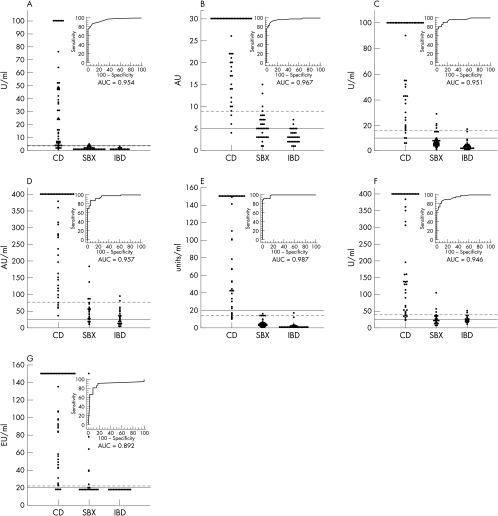
The use of human tTG (h-tTG), versus guinea pig liver tTG (gpl-tTG), in these assays has been reported to supply superior outcomes. This examine in contrast 13 business IgA tTG ELISA kits to establish their efficiency traits within the analysis of CD in sufferers with biopsy confirmed illness in contrast with controls.
All sufferers and controls had been adults aged 21 years or older.METHODSSera from the next teams of sufferers had been examined in every package: (1) 49 sufferers with CD confirmed on small bowel biopsies (all IgA EMA optimistic); (2) 34 sufferers with small bowel biopsies that weren’t in line with CD; and (3) 30 sufferers with biopsy confirmed inflammatory bowel illness.
All controls had been unfavourable for IgA EMA and weren’t IgA poor. Sensitivities and specificities had been decided utilizing each the producers’ really useful lower off factors and receiver working attribute (ROC) evaluation derived determination thresholds.
The space underneath the curve (AUC) for every ROC plot was additionally calculated and in contrast between kits.RESULTSIn basic, the h-tTG based mostly IgA tTG ELISA kits demonstrated superior efficiency (particularly specificity) in contrast with the gpl-tTG based mostly kits, though 100% sensitivity and specificity (akin to the IgA EMA assay) was obtained in just one recombinant h-tTG based mostly package.
CONCLUSIONSThe use of h-tTG in IgA tTG ELISA kits is mostly, however not universally, related to superior efficiency. Factors aside from antigen supply are vital in figuring out package efficiency.
A new ELISA package which makes use of a mix of Plasmodium falciparum extract and recombinant Plasmodium vivax antigens as a substitute for IFAT for detection of malaria antibodies.
BACKGROUNDThe strategies mostly used to measure malarial antibody titres are the Indirect Fluorescence Antibody Test (IFAT), thought to be the gold customary, and the Enzyme-Linked ImmunoSorbent Assay (ELISA).
The goal right here was to evaluate the diagnostic efficiency, i.e. the sensitivity and specificity, of a brand new malaria antibody ELISA package in comparison to IFAT.
This new ELISA package, the ELISA malaria antibody take a look at (DiaMed), makes use of a mix of crude soluble Plasmodium falciparum extract and recombinant Plasmodium vivax antigens.
METHODSTwo teams had been used: 95 samples from malaria sufferers to evaluate the medical sensitivity and 2,152 samples from blood donors, who had not been uncovered to malaria, to evaluate the medical specificity.RESULTSThe DiaMed ELISA take a look at package had a medical sensitivity of 84.2% and a medical specificity of 99.6% as in contrast with 70.5% and 99.6% respectively, utilizing the IFAT technique. The ELISA technique was extra delicate than the IFAT technique for P.
vivax infections (75% vs. 25%). However, in 923 malaria danger donors the analytical sensitivity of the ELISA take a look at was 40% and its specificity 98.3%, performances impaired by giant numbers of equivocal outcomes non-concordant between ELISA and IFAT.
When the general analytical performances of ELISA was in comparison with IFAT, the ELISA effectivity J index was 0.84 versus 0.71 for IFAT. Overall analytical sensitivity was 93.1% and the analytical specificity 96.7%. Overall settlement between the 2 strategies reached 0.97 with a reliability okay index of 0.64.
CONCLUSIONSThe DiaMed ELISA take a look at package reveals an excellent correlation with IFAT for analytical and medical parameters. It could also be an attention-grabbing technique to switch the IFAT particularly in blood banks, however additional intensive investigations are wanted to study the analytical efficiency of the assay, particularly in a blood financial institution setting.
