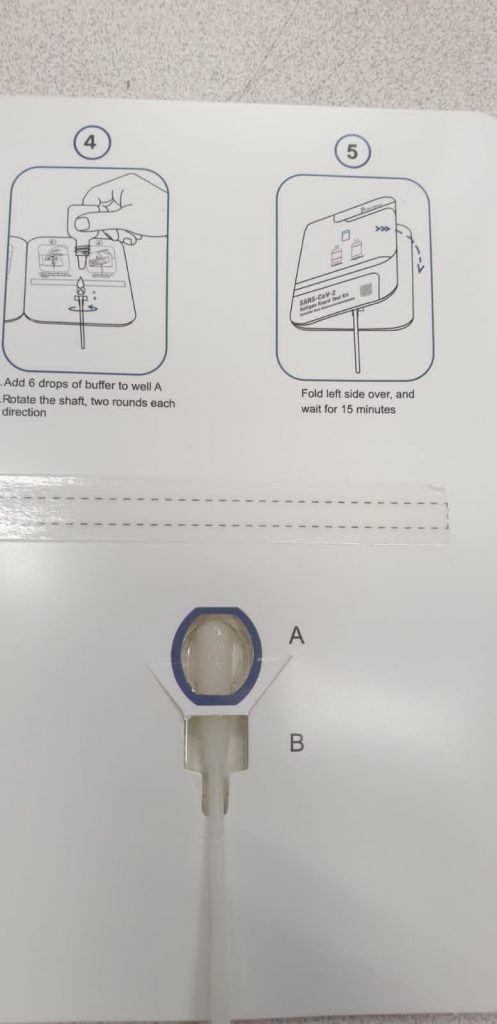
Comparison of infliximab drug measurement across three commercially available ELISA kits.
The monitoring of infliximab drug levels aids in the management of several autoimmune diseases, notably inflammatory bowel disease. Several commercial kits are now available and approved by the Therapeutic Goods Administration (TGA) for the measurement of infliximab levels, but there have been limited verification or comparison studies to date. Finding an assay that most accurately […]